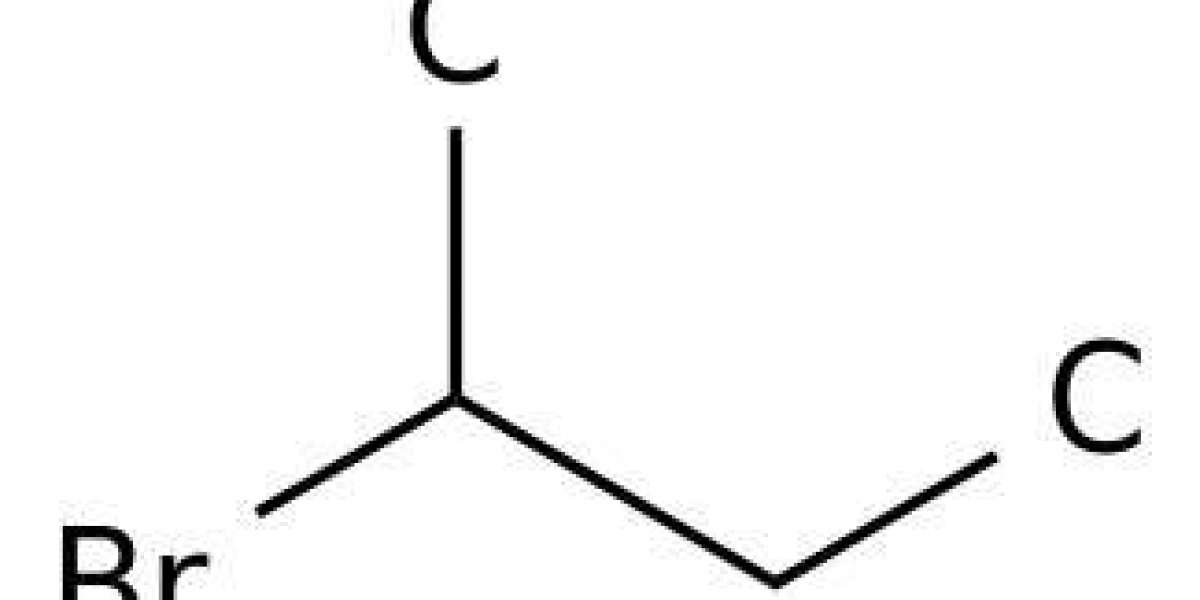
Rotational spectra and conformational analysis of 2 bromobutane
The rotational spectra of 2 bromobutane were obtained by using a chirped-pulse Fourier-transform microwave spectrometer in the frequency range 8–18 GHz. Hyperfine structures of both bromine isotopologues (I = 3/2) for the three conformers have been investigated to determine the rotational constants, centrifugal distortion constants, and nuclear quadrupole coupling constants of bromine (Br). The conformational compositions for G+, A, and G− in the supersonic jet, estimated by relative intensity measurements, are 55%, 28%, 17%, respectively, for the 79Br species, and 52%, 33%, 15%, respectively, for the 81Br species. However, the small zero-point energy differences of isotopomers can be negligible and can be considered as an average value instead of individual values, which is 54%, 30%, 16% for G+, A, and G− species. The nuclear quadrupole coupling tensors of Br and the quadrupolar angles were obtained, and the χzz value of 2 bromobutane is compared with those of other alkyl bromides. The spectroscopic constants, rs coordinates of Br and the dipole moments of the most stable G+ conformer are predicted well from the ab initio calculations at the MP2/6–311++G(2d,2p) level.
Conformational analyses of 2-halobutanes have previously been conducted by using infrared spectroscopy, Raman spectroscopy, microwave spectroscopy, electron diffraction analysis, and theoretical calculations. In this paper, according to the dihedral angle of the Br-C2-C3-C4 bonds, the three conformers of 2 bromobutane are labeled as A (anti), G+, and G− (gauche), corresponding to dihedral angles of ∼180 °, +60 °, and −60 °, respectively.
To the best of our knowledge, rotational isomerism of 2 bromobutane was first studied by determining the enthalpy differences and potential energy barriers of the transition states between the isomers using IR and ultrasonic absorption measurements; the G+ isomer was found to be the lowest-energy one. Another IR spectroscopic study of the crystalline and glassy states revealed that crystalline 2-halobutane also adopts the G+ conformation, i.e. the zig-zag planar carbon chain conformation. Consistent results were obtained from a Raman spectroscopic study on conformation changes in the pure liquid state. In earlier theoretical studies, empirical calculations of conformational energies predicted that the gas-phase composition of a 2-halobutane at 300 K was G + 65%, A 15%, and G − 20%. In a recent study using gas electron diffraction experiments, the radial distribution curves of the ab initio calculated conformational compositions for 2 bromobutane did not fit the experimental RD-curves; thus, modified compositions were reported by specifying the A conformer content as only 10% and using the relative energies for the two gauche conformers. On the other hand, the ab initio calculated composition of 2-chlorobutane fitted quite well with the experimental curves, and the refined composition was obtained with high uncertainties: G + 72(41)%, A 17(11)%, G − 11(42)%. Since relative intensity measurements in microwave spectroscopy can be used to estimate the relative populations of 2 bromobutane conformers in the supersonic jet, herein we have attempted to extract this information.
Compared to other halogen atoms, bromine has nearly identical isotopic abundances for 79Br 50.69(7)% and 81Br 49.31(7)%; this produces “double” peaks of equal intensity in spectroscopic experiments. For example, the paired signals were recently reported in the photodissociation measurements for 2 bromobutane, with approximately identical intensities owing to the presence of two equal-abundance Br isotopes. In this study, rotational transitions and hyperfine structures (I = 3/2 for Br) for the three conformers and the two Br isotopologues of 2 bromobutane have been obtained using microwave spectroscopy, and assigned to spectroscopic constants comparable with the ab initio calculations. Conformational compositions and structural parameters for the three conformers have been discussed, and the dipole moment components of the G+ conformer have been determined based on Stark shift observations.