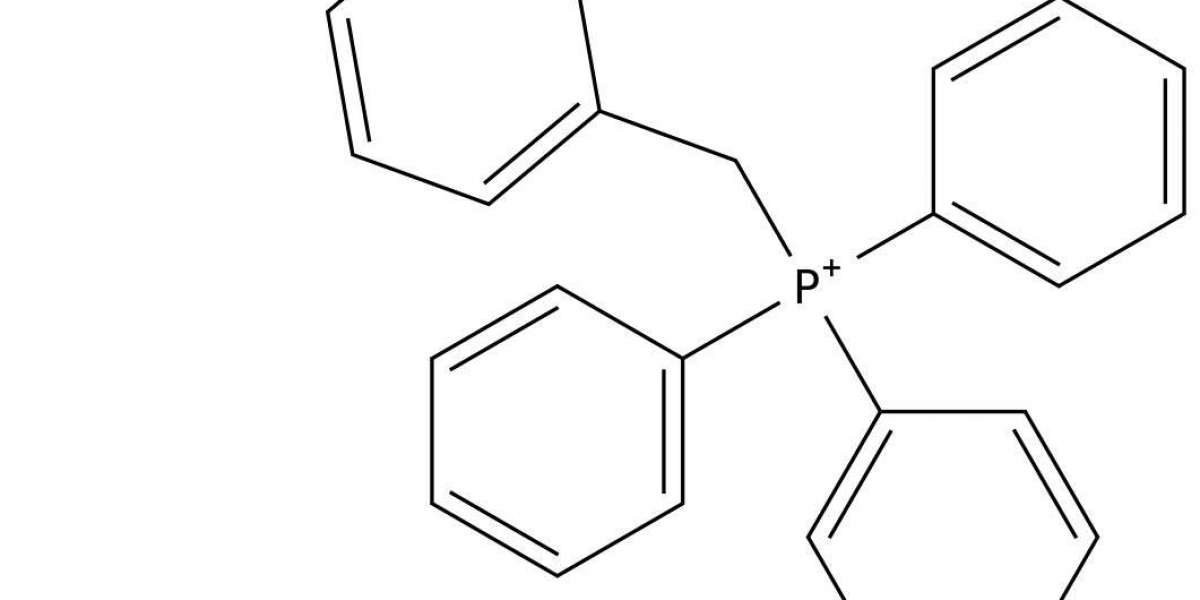
Synergistic Inhibition between Benzyltriphenylphosphonium Chloride and Halide Ions on the Corrosion of Mild Steel in Acidic Medium
The effect of Benzyltriphenylphosphonium Chloride (BTPPC) and halide ion (KI) on the corrosion of mild steel in a solution of 0.3 M phosphoric acid have been investigated at various inhibitor concentrations and temperatures by potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), temperature kinetic, scanning electron microscopy (SEM) and atomic force microscopy (AFM) studies, respectively. Results obtained from potentiodynamic polarization studies, reveal that BTPPC and KI are mixed type inhibitors for mild steel in 0.3 M phosphoric acid.
The synergistic effect of BTPPC and KI in corrosion inhibition of mild steel in 0.3 M H3PO4 containing low concentration of I- has been evaluated by potentiodynamic polarization studies. The experimental results suggest that the presence of iodide ions in the solution stabilized the adsorption of BTPPC molecules on the metal surface and improved the inhibition efficiency of BTPPC. The corrosion behavior of mild steel in 0.3 M H3PO4 without and with the inhibitor at various concentrations was studied in the temperature range from (298 to 338) K.
The inhibition efficiency increases with an increase in concentration at all temperatures. The inhibition efficiencies decrease with an increase in temperature. The adsorption of BTPPC + KI accords to the Temkin adsorption isotherm. Kinetic and thermodynamic parameters such as effective activation energy (Ea), Gibbs free energy of adsorption (AGo ads) and heat of adsorption (AHoads) indicate that the adsorption of BTPPC + KI on the mild steel surface is primarily physical in nature.
The results of scanning electron microscopy and atomic force microscopy are in agreement with the electrochemical analysis results. The β phase of polyvinylidene fluoride (PVDF) crystals is polar and has very good piezoelectric and dielectric properties as compared with the nonpolar α phase. Benzyltriphenylphosphonium Chloride (BTPC) has been previously shown to directly nucleate the β phase from melt, instead of α phase, and is an additive of practical importance. Different amounts of BTPC were melt mixed into PVDF using a micro twin screw extruder to study the rheology of the blends using oscillatory and steady shear viscometry. Data at different temperatures were found to superimpose onto a master curve using time-temperature superposition. The complex viscosity and steady shear viscosity increased significantly upon addition of 0.5% BTPC and decreased slightly with further addition of BTPC. The storage modulus exhibited a plateau at low frequencies indicating structure formation in the melt on addition of BTPC.
The horizontal shift factors derived from the time-temperature superposition were found to follow an Arrhenius temperature dependence and the flow activation energy for each blend was obtained. Pure PVDF and PVDF films with 1% and 3% BTPC were melt extruded using a laboratory twin screw extruder. The film containing 3% of BTPC gave the highest fraction of β phase crystals (75%). Small angle light scattering results showed that the size of spherulites decreased with increase in the weight fraction of BTPC. The dielectric constant and conductivity of the films at low frequencies increased significantly with concentration of BTPC, as did the dielectric loss and AC electrical conductivity.